SEND is one of the required standards for data submission to the FDA. What role could AI play in the context of SEND?
With the rapid growth of AI across various industries, it is inevitable that our biotech industry needs to continue to embrace forthcoming innovation and progress accordingly. AI is powered by data for which nonclinical safety and toxicological assessments produce a significant amount. In our previous blog on whether ...to SEND or not to SEND.., we touched on how the use of SEND enhances data review and enables data warehousing, data analysis and data visualization in addition to having a set of controlled terminology for the biotech industry as a whole.
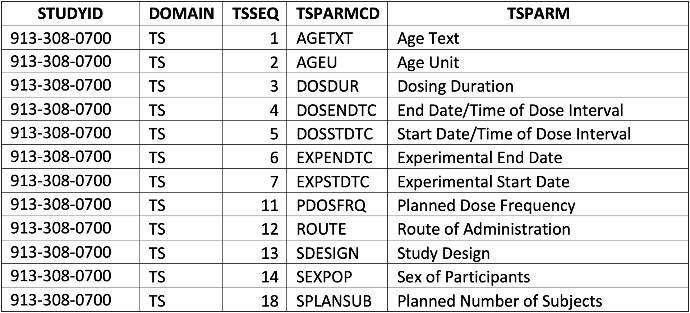
The multiple domains set by the FDA in its guidelines for SEND holds huge promise for generative AI purposes. Data from nonclinical studies are large and diverse, therefore correlations from multiple studies, treatment groups, sexes, time points, and endpoints are not easily digestible if data is presented only as group means and standard deviations. The question then becomes, will computer Application Programming Interfaces along with AI facilitate deeper review and understanding? By examining subsets of a SEND dataset package, we can begin to uncover the answer to that question. For example, the Trial Summary (TS) domain shows details (sample snippet below) of study information available within a SEND dataset. Such structured data is instrumental in enabling the extraordinary potential of AI.
As AI becomes more accessible, we see the nonclinical research scientist becoming more dynamic and having more easily accessible tools to review and make informed decisions on large datasets such as those obtained from SEND. Attentive Science uses industry standard data acquisition systems which allows conversion of research data to SEND formats in an expedient manner. Our in-house data specialist works directly with our Study Directors each step of the way to ensure the SEND data conversion is smooth.
Is it time for us to be thinking of ourselves as information technology entities being attentive to science?
The time has come for us all to take a leaf out of the books of “Techbio” companies such as Insilico Medicine, Exscientia, and Recursion who have actively been proving the AI hypothesis. Bioinformatics personnel at CRO’s and pharma biotech companies are going to be vital. We predict an augmented use of AI in reviewing SEND datasets and making the benefits of AI in nonclinical in-vivo research more commercially available. Watch this state of the ARTspace!
If you are planning an IND, or in the midst of one and have locked in date(s) for submission, there is no time to waste. Call us and we will put our team behind your efforts.